Bronchial asthma, chronic bronchitis, emphysema and other lung disease with acute bronchospasm.
Specifications
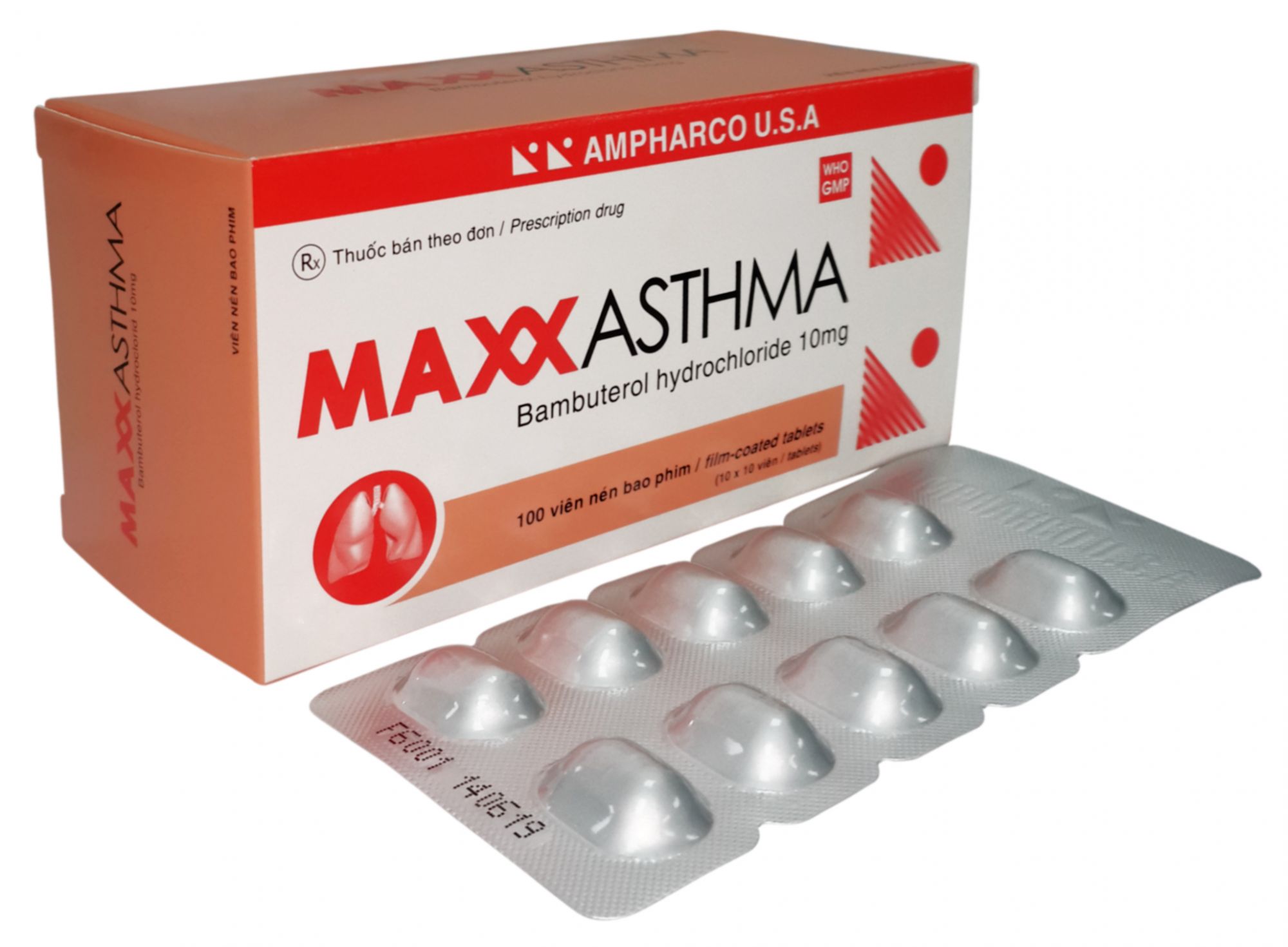
MAXXASTHMA
COMPOSITION
Each film-coated tablet contains:
Bambuterol hydrochloride......................... 10 mg
Excipients: Lactose, microcrystalline cellulose, magnesium stearate, opadry II white.
INDICATIONS
Bronchial asthma, chronic bronchitis, emphysema and other lung disease with acute bronchospasm.
DOSAGE AND ADMINISTRATION
Bambuterol should be used as maintenance therapy in asthma and other pulmonary diseases where bronchospasm is a complicating factor.
Recommended dose is once daily, preferably shortly before bedtime. The dose should be individualised.
Adults: The recommended initial dose is 10 mg. The dose may be increased to 20 mg after 1–2 weeks, depending on the clinical effect. In patients who have previously tolerated oral β2-agonists well, the recommended initial dose is 20 mg.
Elderly: dosage as adults, dose adjustment is not required.
Significant hepatic dysfunction: Not recommended because of unpredictable conversion to terbutaline.
In patients with a moderate to severely impaired renal function (GFR ≤ 50 ml/min): the recommended initial dose is 5 mg, which may be increased to 10 mg after 1–2 weeks, depending on the clinical effect.
Paediatric population: Until the clinical documentation has been completed, bambuterol should not be used in children.
CONTRAINDICATIONS
Hypersensitivity to terbutaline or any ingredients in the formulation.
Bambuterol is presently not recommended for children due to limited clinical data in this age group.
WARNING AND PRECAUTIONS
As terbutaline is excreted mainly via the kidneys, the dose of bambuterol should be halved in patients with an impaired renal function (GFR ≤ 50 mL/min).
In patients with liver cirrhosis, and probably in patients with other causes of severely impaired liver function, the daily dose must be individualized, taking into account the possibility that the individual patient could have an impaired ability to metabolise bambuterol to terbutaline. Not recommended for patients with severe hepatic impairment because of unpredictable conversion to terbutaline.
As for all β2-agonists, caution should be observed in patients with thyrotoxicosis and in patients with severe cardiovascular disorder, such as ischemic heart disease, tachyarrhythmias or severe heart failure.
Due to the hyperglycemic effects of β2-agonists, additional blood glucose controls are recommended initially in diabetic patients.
Due to the positive inotropic effects of β2-agonists, these drugs should not be used in patients with hypertrophic cardiomyopathy.
Potentially serious hypokalemia may result from β2-agonist therapy. Particular caution is recommended in acute severe asthma as the associated risk may be augmented by hypoxia. The hypokalemic effect may be potentiated by concomitant treatments (see DRUG INTERACTION section). It is recommended that serum potassium levels are monitored in such situations.
Precaution should be applied when treating patients predisposed to angle closure glaucoma.
Pregnancy
Although no teratogenic effects have been observed in animals after administration of bambuterol, caution is recommended during the first trimester of pregnancy.
Transient hypoglycaemia has been reported in newborn pre-term infants after maternal β2-agonist treatment.
Lactation
It is unknown whether bambuterol or intermediary metabolites are excreted in human breast milk. Terbutaline is excreted in breast milk, but an influence on the child is unlikely with therapeutic doses.
PRESENTATION
Blister of 10 film-coated tablets. Box of 3 blisters.
Keep out of reach of children
Read the package insert carefully before use
Ask your doctor for further information
Use upon doctor’s prescription only
Manufactured and Distributed by: AMPHARCO U.S.A PHARMACEUTICAL JOINT-STOCK COMPANY
Nhon Trach 3 Industrial Park, Hiep Phuoc Ward, Nhon Trach District, Dong Nai Province
Tel: 02513 566 202 - Fax: 02513 566 203