- Treatment of hypertension.
- Treatment of chronic stable angina pectoris.
- Treatment of stable chronic heart failure with reduced systolic left ventricular functionin addition to ACE inhibitors and diuretics, and optionally cardiac glycosides.
Specifications
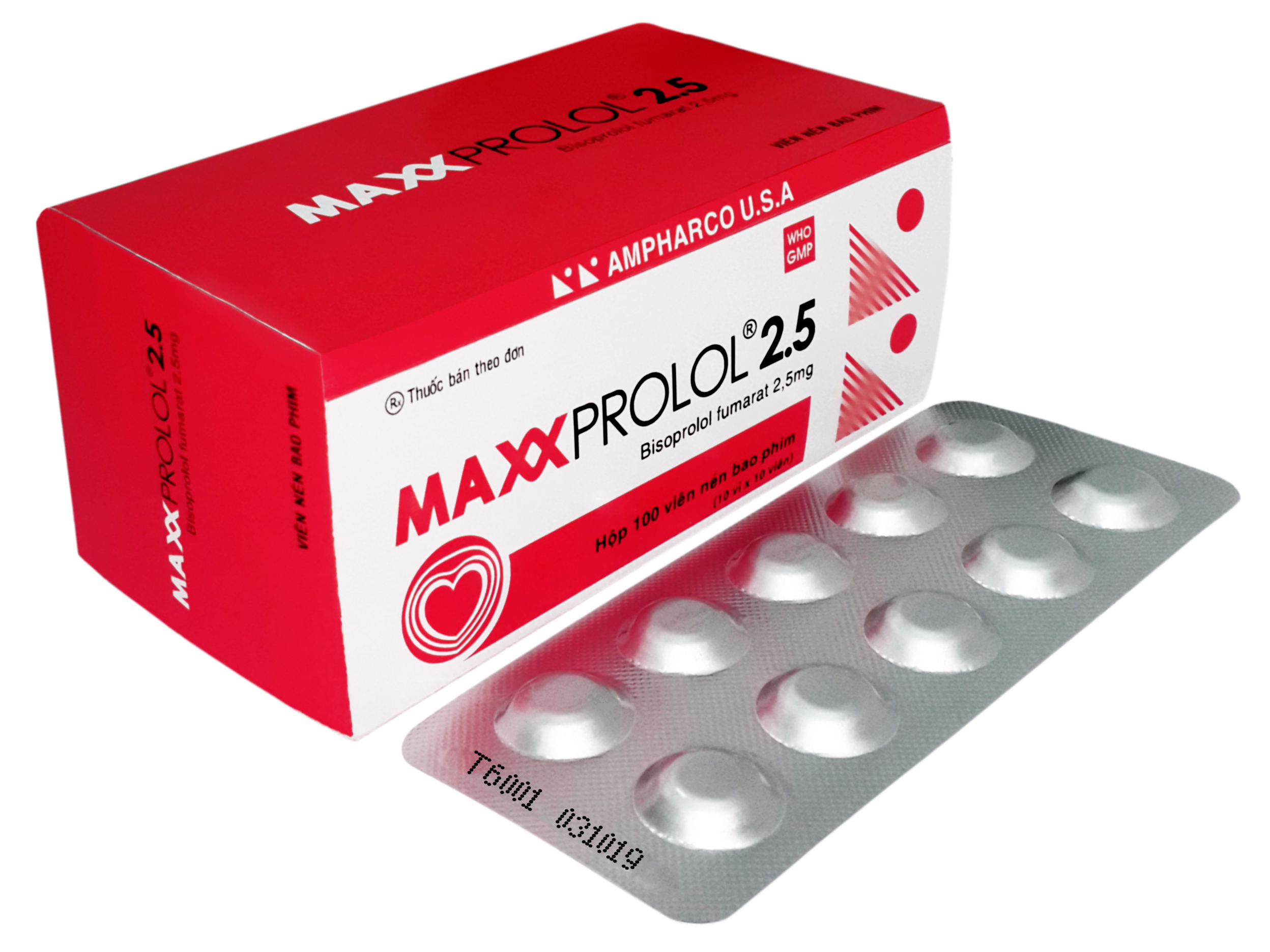
MAXXPROLOL®2.5
COMPOSITION: Each film-coated tablet contains:
Bisoprolol fumarate................................................... .. 2.5 mg
Excipients: Pregelatinised starch, Calcium hydrogen phosphate anhydrous, Microcrystalline cellulose, Colloidal anhydrous silica, Magnesium stearate, Opadry II pink, Sunset yellow lake.
INDICATIONS:
- Treatment of hypertension.
- Treatment of chronic stable angina pectoris.
- Treatment of stable chronic heart failure with reduced systolic left ventricular functionin addition to ACE inhibitors and diuretics, and optionally cardiac glycosides.
DOSAGE AND ADMINISTRATION:
Bisoprolol tablets should be taken in the morning and can be taken with food. They should be swallowed with liquid and should not be chewed.
Treatment of hypertension and chronic stable angina pectoris
Adults: the dosage should be individually adjusted. It is recommended to start with 5 mg per day. The usual dose is 10 mg once daily with a maximum recommended dose of 20 mg per day.In milder forms of hypertension (diastolic blood pressure up to 105 mmHg) therapy with 2.5 mg once daily may be adequate.
Patients with bronchospastic disease: bisoprolol fumarat 2.5 mg may be an appropriate starting dose.
Patients with renal impairment: in patients with severe renal impairment (creatinine clearance < 20 ml/min) the dose should not exceed 10 mg once daily. This dosage may eventually be divided into halves.
Patients with severe liver impairment: no dosage adjustmentis required, however careful monitoring is advised.
Elderly: no dosage adjustment is normally required. It is recommended to start with the lowest possible dose.
Children: there is no experience with bisoprolol in children, therefore its use cannot be recommended for children.
Discontinuation of treatment: treatment should not be stopped abruptly. The dosage should be diminished slowly by a weekly halving of the dose.
Treatment of stable chronic heart failure (CHF)
Adults: standard treatment of CHF consists of an ACE inhibitor (or an angiotensin receptor blocker in case of intolerance to ACE inhibitors), a beta-blocker, diuretics, and when appropriate cardiac glycosides. Patients should be stable (without acute failure) when bisoprolol treatment is initiated.It is recommended that the treating physician should be experienced in the management of chronic heart failure.Transient worsening of heart failure, hypotension, or bradycardia may occur during the titration period and thereafter.
Titration phase: the treatment of stable chronic heart failure with bisoprolol requires a titration phase.
The treatment with bisoprolol is to be started with a gradual uptitration according to the following steps:
- 25mg once daily for 1 week,if well tolerated increase to
- 5 mg once daily for a further week,if well tolerated increase to
- 75 mg once daily for a further week, if well tolerated increase to
- 5 mg once daily for 4 following weeks, if well tolerated increase to
- 5 mg once daily for 4 following weeks, if well tolerated increase to
- 10 mg once daily for the maintenance therapy.
The maximum recommended dose is 10 mg once daily.
Close monitoring of vital signs (heart rate, blood pressure) and symptoms of worsening heart failure is recommended during the titration phase. Symptoms may already occur within the first day after initiating the therapy.
In some patients, bisoprolol fumarate 2.5 mg may be sufficient for maintenance therapy.
Treatment modification: if the maximum recommended dose is not well tolerated, gradual dose reduction may be considered.In case of transient worsening of heart failure, hypotension, or bradycardia reconsideration of the dosage of the concomitant medication is recommended. It may also be necessary to temporarily lower the dose of bisoprolol or to consider discontinuation.The reintroduction and/or uptitration of bisoprolol should always be considered when the patient becomes stable again.If discontinuation is considered, gradual dose decrease is recommended, since abrupt withdrawal may lead to acute deterioration of the patient condition.
Treatment of stable chronic heart failure with bisoprolol is generally a long-term treatment.
Special population
Renal or hepatic impairment: there is no information regarding pharmacokinetics of bisoprolol in patients with chronic heart failure and with impaired hepatic or renal function. Uptitration of the dose in these populations should therefore be made with additional caution.
Elderly: no dosage adjustment is normally required.
Children: there is no paediatric experience with bisoprolol, therefore its use cannot be recommended for children.
CONTRAINDICATIONS:
MAXXPROLOL®is contraindicated in patients with:
- Acute heart failure or during episodes of heart failure decompensation requiring i.v. inotropic therapy.
- Cardiogenic shock.
- Second or third degree AV block (without a pacemaker).
- Sick sinus syndrome.
- Sinoatrial block.
- Bradycardia (< 60 beats/min) before the start of therapy.
- Hypotension (systolic blood pressure less than 100 mm Hg).
- Severe bronchial asthma or severe chronic obstructive pulmonary disease.
- Late stages of peripheral arterial occlusive disease and Raynaud's syndrome.
- Untreated phaeochromocytoma.
- Metabolic acidosis.
- Hypersensitivity to bisoprolol or to any of the excipients.
WARNINGS AND PRECAUTIONS:
Cardiac failure: sympathetic stimulation is avital component supportingcirculatoryfunction in the setting of congestive heart failure, and beta-blockade may result in furtherdepression of myocardial contractility and precipitate more severe failure. In general, beta-blocking agents should be avoided in patients with overt congestive failure. However, in some patients with compensated cardiac failure it may be necessary to utilize them. In such a situation, they must be used cautiously.
In patients without a history of cardiac failure: continued depression of the myocardium with beta-blockers can, in some patients, precipitate cardiac failure. At the first signs or symptoms ofheart failure, discontinuation of bisoprolol fumarate should be considered. In some cases, beta-blockertherapycan be continued whileheartfailure is treated with other drugs.
Abrupt Cessation of Therapy: exacerbationofangina pectoris, and, in some instances,myocardial infarctionorventricular arrhythmia, have been observed in patients with coronaryarterydisease following abrupt cessation of therapy with beta-blockers. Such patients should, therefore, be cautioned against interruption or discontinuation of therapy without the physician's advice. Even in patients without overt coronary artery disease, it may be advisable to taper therapy with bisoprolol fumarate over approximately one week with the patient under careful observation. If withdrawal symptoms occur, bisoprolol fumarate therapy should be reinstituted, at least temporarily.
Peripheral Vascular Disease: beta-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease. Caution should be exercised in such individuals.
Bronchospastic Disease: patients with bronchospastic disease should, in general, not receive beta-blockers. Because of its relative beta1-selectivity, however, bisoprolol fumarate may be used with caution in patients with bronchospastic disease who do not respond to, or who cannot tolerate other antihypertensive treatment. Since beta1-selectivity is not absolute, the lowest possible dose of bisoprolol fumarate should be used, with therapy starting at 2.5 mg. A beta2 agonist (bronchodilator) should be made available.
Major Surgery: chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond toreflexadrenergicstimuli may augment the risks of generalanesthesia and surgical procedures.
Diabetes and Hypoglycemia: beta-blockers may mask some of the manifestations of hypoglycemia, particularlytachycardia. Nonselective beta-blockers may potentiate insulin-induced hypoglycemia and delay recovery of serum glucose levels. Because of its beta1-selectivity, this is less likely with bisoprolol fumarate. However, patients subject to spontaneous hypoglycemia, or diabetic patients receivinginsulinor oralhypoglycemic agents, should be cautioned about these possibilities and bisoprolol fumarate should be used with caution.
Thyrotoxicosis: beta-adrenergic blockade may mask clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of beta-blockade may be followed by an exacerbation of the symptoms of hyperthyroidism or may precipitatethyroid storm.
Safety in pregnancy: bisoprolol (beta-blockers) reduces placental perfusion which has been associated with intrauterine growth retardation, intrauterine death, abortion or early labour. Adverse effects (e.g. hypoglycaemia and bradycardia) may occur in the foetus and the new-born infant. If treatment with a beta-blocker is necessary, beta1-selective beta-blockers are preferable. Bisoprolol is not recommended during pregnancy unless clearly necessary.
If treatment with bisoprolol is considered necessary, utero-placental blood flow and foetal growth must be monitored. In the case of harmful effects on pregnancy or the foetus, alternative therapeutic measures should be considered. The new-born infant must be monitored closely. Symptoms of hypoglycaemia and bradycardia are generally to be expected within the first 3 days of life.
Safety in breast feeding: it is not known whether bisoprolol is excreted in human breast milk. Therefore, breastfeeding is not recommended during bisoprolol therapy.
PRESENTATION: Aluminium - Aluminium blister. Blister of 10 film-coated tablets.Box of 1, 3, or 10 blisters.
Keep out of reach of children
Read the package insert carefully before use
Ask your doctor for further information
Use upon doctor’s prescription only
Manufactured and Distributed by: AMPHARCO U.S.A PHARMACEUTICAL JOINT-STOCK COMPANY
Nhon Trach 3 Industrial Park, Hiep Phuoc Ward, Nhon Trach District, Dong Nai Province
Tel: 02513 566 202 - Fax: 02513 566 203