- Glimepiride is indicated as an adjunct to proper dietary management, exercise and weight reduction to lower the blood glucose in patients with type 2 diabetes whose hyper-glycemia cannot be controlled by diet and exercise alone.
- Glimepiride may be used in combination with metformin or insulin.
Specifications
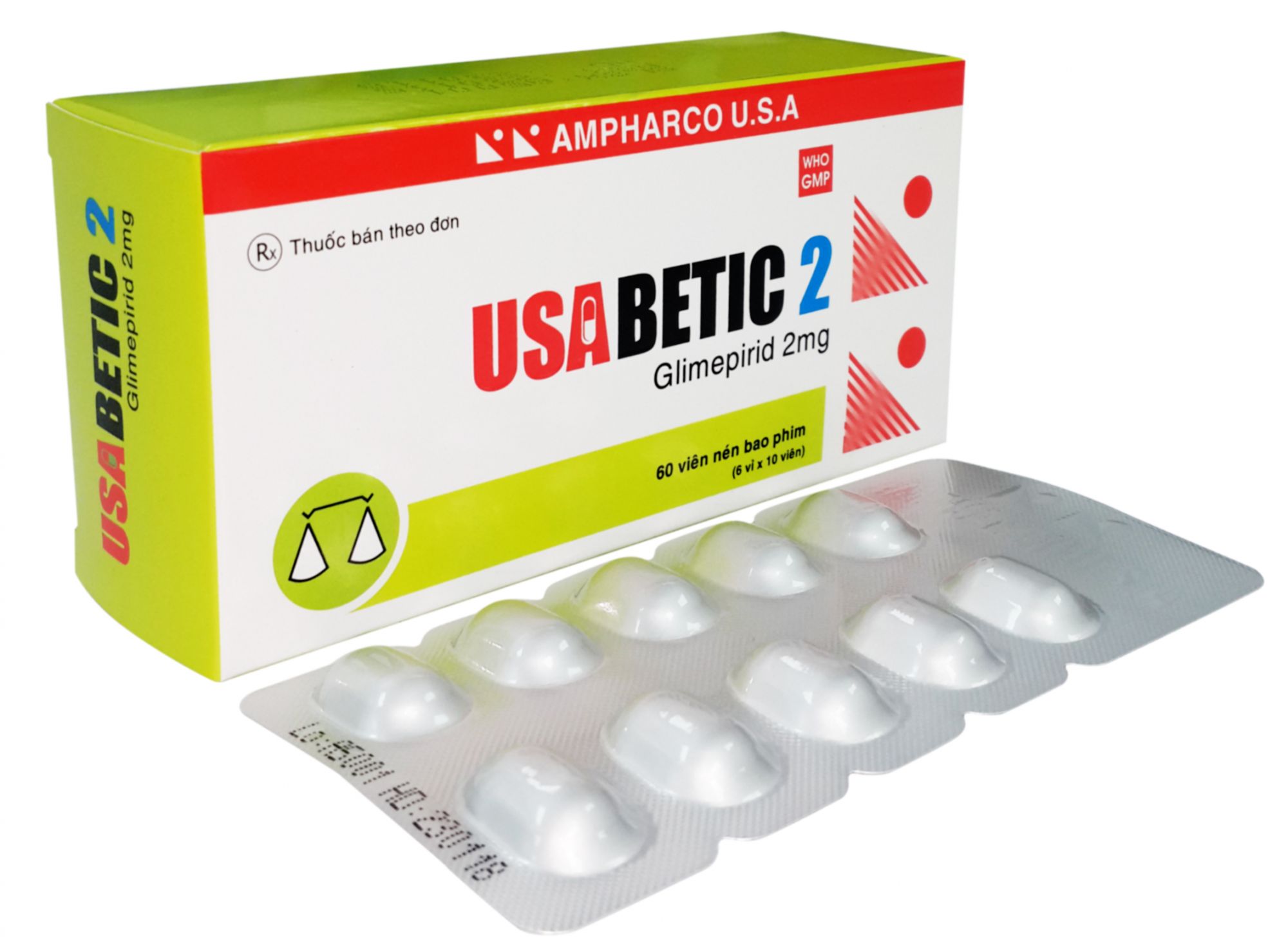
USABETIC 2
COMPOSITION: Each film-coated tablet contains:
- Glimepiride……………………………………2 mg
- Excipients: Lactose, Microcrystalline cellulose, Sodium starch glycolate, Magnesium stearate, Opadry II white, Brilliant blue.
INDICATIONS:
- Glimepiride is indicated as an adjunct to proper dietary management, exercise and weight reduction to lower the blood glucose in patients with type 2 diabetes whose hyper-glycemia cannot be controlled by diet and exercise alone.
- Glimepiride may be used in combination with metformin or insulin.
DOSAGE AND ADMINISTRATION
Dosage must be individualized based on patient’s glycemia.
Usual dose: 1 - 4 mg; taken once at breakfast.
The maximum recommended dose is 8 mg once daily.
Dose adjustment is necessary in impaired hepatic or renal function
CONTRAINDICATIONS:
- History of hypersensitivity to glimepiride and other sulfonylureas or any other component of the formulation.
- Insulin dependent diabetes mellitus (type 1).
- Diabetic ketoacidosis
- Diabetic coma or pre-coma.
- Severe kidney or liver failure.
- Pregnant or planning pregnant women, lactating women.
- Severe infection, trauma, major surgery.
WARNINGS AND PRECAUTIONS:
Use of glimepiride must be considered as treatment in addition to a proper dietary regimen and not as a substitute for diet. Proper dietary management and exercise alone may be effective in controlling the blood glucose and symptoms of hyperglycemia.
Elderly, debilitated or malnourished patients, and those with adrenal, pituitary, or hepatic insufficiency are particularly susceptible to the hypoglycemic action of glucose-lowering drugs. Patients with impaired renal function may be more sensitive to the glucose-lowering effect of glimepiride.
When a patient stabilized on any diabetic regimen is exposed to stress such as illness during therapy, fever, trauma, infection, or surgery, a loss of glycemic control may occur. At such times, it may be necessary to adjust the dosage of glimepiride, add insulin in combination with glimepiride or even use insulin monotherapy.
Hypoglycemia may be difficult to recognize in the elderly and in people who are taking beta-adrenergic blocking drugs or other sympatholytic agents.
The effectiveness of any oral hypoglycemic drug, including glimepiride, in lowering blood glucose to a desired level decreases in many patients over a period of time, which may be due to progression of the severity of the diabetes or to diminished responsiveness to the drug. This phenomenon is known as secondary failure. Should secondary failure occur with glimepiride or metformin monotherapy, combined therapy with glimepiride and metformin or glimepiride and insulin may result in a response. Should secondary failure occur with combined glimepiride /metformin therapy, it may be necessary to initiate insulin therapy.
Fasting blood glucose should be monitored periodically and HbA1C should be monitored every 3-6 months to determine therapeutic response.
Pregnant Women:
There are no adequate and well-controlled studies in pregnant women. On the basis of results from animal studies, glimepiride should not be used during pregnancy.
Patients who are planning a pregnancy should consult their physician, and it is recommended that they change over to insulin for the entire course of pregnancy and lactation.
Nursing Mothers:
Glimepiride is excreted in human milk. Therefore, it should be discontinued in nursing mothers, insulin therapy should be considered.
PRESENTATION: Blister of 10 film-coated tablets, box of 6 blisters.
Keep out of reach of children
Read the package insert carefully before use
Ask your doctor for further information
Use upon doctor’s prescription only
Manufacturer and Distributed by: AMPHARCO U.S.A PHARMACEUTICAL JOINT-STOCK COMPANY
Nhon Trach 3 Industrial Park, Hiep Phuoc, Nhon Trach, Dong Nai.
Tel: 02513 566 202 - Fax: 02513 566 203