- Asthma:Montelukast is indicated for the prophylaxis and chronic treatment of asthma in adults and pediatric patients 12 months of age and older.
- Exercise-Induced Bronchoconstriction (EIB): Montelukastis indicated for prevention of exercise-induced broncho-constriction (EIB) in patients 6 years of age and older.
- Allergic Rhinitis: Montelukast is indicated for the relief of symptoms of seasonal allergic rhinitis in patients 2 years of age and older and perennial allergic rhinitis in patients 6 months of age and older.
Specifications
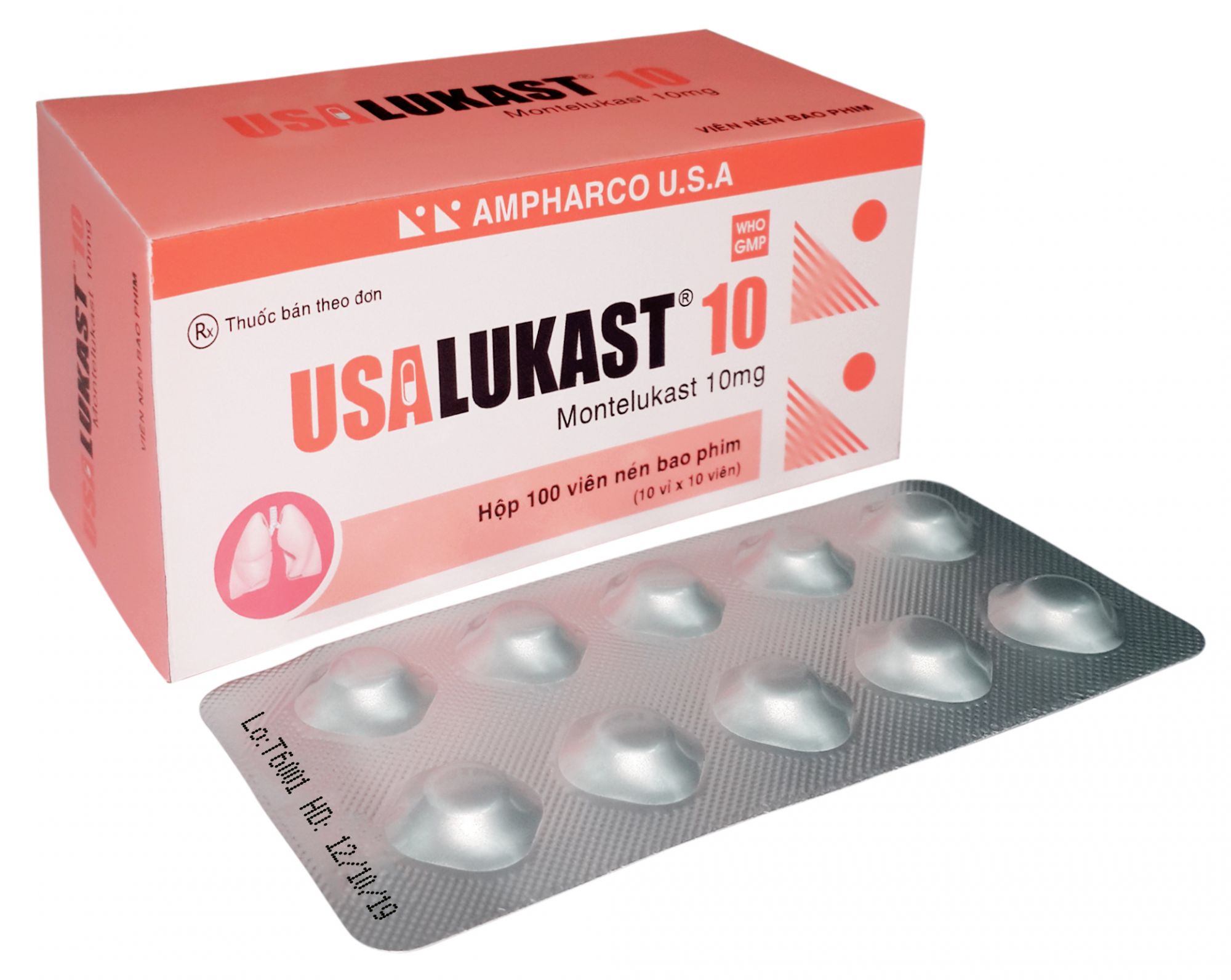
USALUKAST®10
COMPOSITION: Each film-coated tablet contains:
Montelukast (as montelukast sodium)...... 10 mg
Excipients: Microcrystalline cellulose, Lactose monohydrate, Croscarmellose sodium, Colloidal anhydrous silica, Magnesium stearate, Sunset yellow lake, Opadry II pink.
INDICATIONS
- Asthma:Montelukast is indicated for the prophylaxis and chronic treatment of asthma in adults and pediatric patients 12 months of age and older.
- Exercise-Induced Bronchoconstriction (EIB): Montelukastis indicated for prevention of exercise-induced broncho-constriction (EIB) in patients 6 years of age and older.
- Allergic Rhinitis: Montelukast is indicated for the relief of symptoms of seasonal allergic rhinitis in patients 2 years of age and older and perennial allergic rhinitis in patients 6 months of age and older.
DOSAGE AND ADMINISTRATION
Asthma:USALUKAST® should be taken once daily in the evening. The following doses are recommended:
- For adults & adolescents ≥ 15 years old: one 10-mg tablet.
- For pediatric patients 6 to 14 years old: one 5-mg tablet.
Exercise-Induced Bronchoconstriction (EIB):For prevention of EIB, a single dose of USALUKAST® should be taken at least 2 hours before exercise. The following doses are recommended:
- For adults & adolescents ≥ 15 years old: one 10-mg tablet.
- For pediatric patients 6 to 14 years old: one 5-mg tablet.
An additional dose of USALUKAST® should not be taken within 24 hours of a previous dose. Patients already taking USALUKAST® daily for another indication (including chronic asthma) should not take an additional dose to prevent EIB. All patients should have available for rescue a short-acting β-agonist. Safety and efficacy in patients younger than 6 years of age have not been established. Daily administration of USALUKAST® for the chronic treatment of asthma has not been established to prevent acute episodes of EIB.
Allergic Rhinitis
For allergic rhinitis, USALUKAST® should be taken once daily. Efficacy was demonstrated for seasonal allergic rhinitis when montelukast was administered in the morning or the evening without regard to time of food ingestion. The time of administration may be individualized to suit patient needs.
The following doses for the treatment of symptoms of seasonal allergic rhinitis or perennial allergic rhinitis are recommended:
- For adults & adolescents ≥ 15 years old: one 10-mg tablet.
- For pediatric patients 6 to 14 years old: one 5-mg tablet.
Asthma and Allergic Rhinitis
Patients with both asthma and allergic rhinitis should take only one USALUKAST® dose daily in the evening.
- For adults & adolescents ≥ 15 years old: one 10-mg tablet.
- For pediatric patients 6 to 14 years old: one 5-mg tablet.
CONTRAINDICATIONS
Hypersensitivity to any component of this product.
WARNINGS AND PRECAUTIONS
Montelukast is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmaticus. Patients should be advised to have appropriate rescue medication available.
Montelukast should not be abruptly substituted for inhaled or oral corticosteroids.
Patients with known aspirin sensitivity should continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast. Although montelukast is effective in improving airway function in asthmatics with documented aspirin sensitivity, it has not been shown to truncate bronchoconstrictor response to aspirin and other non-steroidal anti-inflammatory drugs in aspirin sensitive asthmatic patients.
Neuropsychiatric events have been reported in adult, adolescent, and pediatric patients taking montelukast. Patients should be instructed to notify their prescriber if these changes occur. Prescribers should carefully evaluate the risks and benefits of continuing treatment with montelukast if such events occur.
Patients with asthma on therapy with montelukast may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients.
Safety in pregnancy
There are no adequate and well-controlled studies in pregnant women. Montelukast should be used during pregnancy only if clearly needed.
Safety in breast feeding
It is not known if montelukast is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when montelukast is given to a nursing mother.
Pediatric Use
The safety and effectiveness in pediatric patients below the age of 12 months with asthma, 6 months with perennial allergic rhinitis, and 6 years with exercise-induced bronchoconstriction have not been established.
Hepatic Insufficiency
No dosage adjustment is required in patients with mild-to-moderate hepatic insufficiency
Renal Insufficiency
No dosage adjustment is recommended in patients with renal insufficiency
PRESENTATION: Blister of 10 tablets; box of 1, 3 or 10 blisters.
Keep out of reach of children
Read the package insert carefully before use
Ask your doctor for further information
Use upon doctor’s prescription only
Manufactured and distributed by: AMPHARCO U.S.A Pharmaceutical Joint-Stock Company
Nhon Trach 3 Industrial Park, Hiep Phuoc Ward, Nhon Trach District, Dong Nai Province
Tel: 02513 566 202 - Fax: 02513 566 203