- Hypertension: It may be used alone or in combination with other antihypertensive
- Nephropathy in Type 2 Diabetic Patients: It is indicated for the treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria in patients with type 2 diabetes and hypertension. In this population, irbesartan reduces the rate of progression of nephropathy.
Specifications
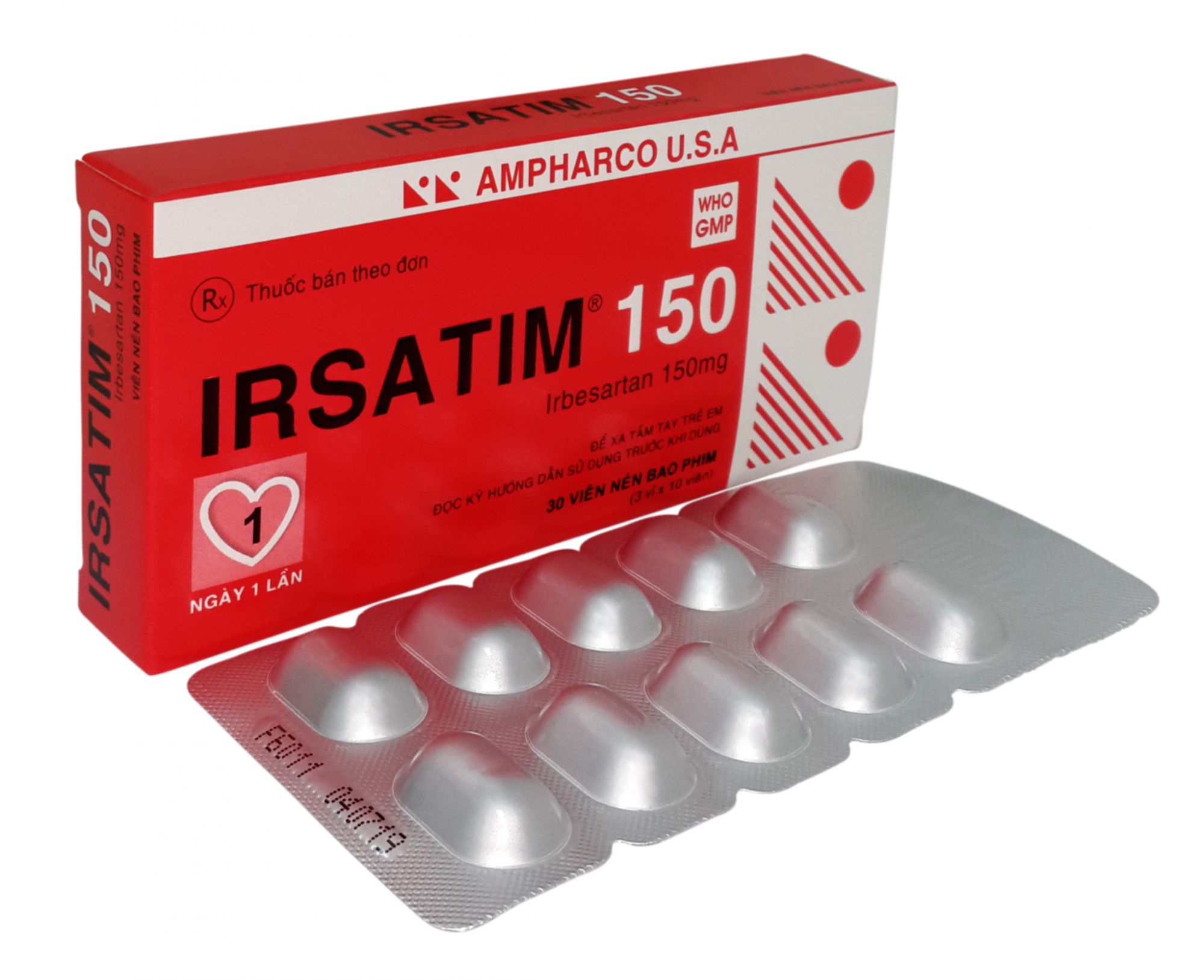
IRSATIM® 150
COMPOSITION: Each film-coated tablet contains:
Irbesartan................................................ 150 mg
Excipients: Lactose, Copovidone, Microcrystalline cellulose, Croscarmellose sodium, Magnesium stearate, Opadry II white, Red iron oxide, Yellow iron oxide, Allura red.
INDICATIONS
- Hypertension: It may be used alone or in combination with other antihypertensive
- Nephropathy in Type 2 Diabetic Patients: It is indicated for the treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria in patients with type 2 diabetes and hypertension. In this population, irbesartan reduces the rate of progression of nephropathy.
DOSAGE AND ADMINISTRATION
Hypertension: The recommended initial dose is 150 mg once daily. Patients requiring further reduction in blood pressure should be titrated to 300 mg once daily. A low dose of a diuretic (hydrochlorothiazide) may be added, if blood pressure is not controlled by irbesartan alone.
No dosage adjustment is necessary in elderly patients, or in patients with hepatic or renal impairment.
Nephropathy in Type 2 Diabetic Patients: The recommended target maintenance dose is 300 mg once daily. There are no data on the clinical effects of lower doses of irbesartan on diabetic nephropathy.
Intravascular volume-and salt-depleted patients: A lower initial dose of irbesartan (75 mg once daily) is recommended in patients with depletion of intravascular volume or salt (eg, patients treated vigorously with diuretics or on hemodialysis).
CONTRAINDICATIONS
- Hypersensitivity to any of its ingredient(s).
- Pregnant women.
- Nursing mothers.
PRECAUTIONS
In patients with intravascular volume- or sodium-depletion, eg, in patients treated vigorously with diuretics, lasting diarrhea or vomiting, such volume depletion should be corrected prior to administration of irbesartan.
In patients with renal artery stenosis, the risk of severe hypotension and renal impairment may be increased when using irbesartan.
Use with caution in renal transplantation or impaired renal function patients. Kalemia and serum creatinine should be controlled frequently.
The risk of hyperkalemia may be increased, especially in patients with impaired cardiovascular or renal function. Kalemia should be tested frequently. Avoid concomitant use of irbesartan with potassium-sparing diuretics.
As other blood vessel dilation drugs, irbesartan has to use with caution in these diseases: aortic valve stenosis and mitral valve stenosis, hypertrophic obstructive cardiomyopathy.
Initiation of antihypertensive therapy may cause symptomatic hypotension in patients with intravascular volume-or sodium-depletion, eg, in patients treated vigorously with diuretics or in patients on dialysis.
Monitor renal function periodically in patients receiving irbesartan and NSAID therapy.
Pediatric Use
Do not use for children under 6 years old since not enough studies have been made until now on these patients.
Irbesartan, in a study at a dose of up to 4.5 mg/kg/day, once daily, did not appear to lower blood pressure effectively in pediatric patients age 6 to 16 years.
Pregnancy: ibersartan is contraindicated for pregnant women. The use of irbesartan is not recommended during the first trimester of pregnancy due to caution. Irbesartan therapy exposure during the second and third trimesters can influence directly on the renin-angiotensin system which induces fetal or neonatal toxicity such as renal failure, skull deformation, even fatal fetal abnormality. When pregnancy is diagnosed, treatment with irbesartan should be stopped immediately. Should exposure to irbesartan have occurred from the second trimester of pregnancy, ultrasound check of renal function and skull is recommended.
Nursing mothers: it is not known whether irbesartan is excreted in human milk, but irbesartan or some metabolite of irbesartan is secreted at low concentration in the milk of lactating rats. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
PRESENTATION: Blister of 10 film-coated tablets. Box of 3 blisters.
Keep out of reach of children
Carefully read the instructions before use
Ask your doctor for further information
Use upon doctor’s prescription only
Manufactured and Distributed by: AMPHARCO U.S.A PHARMACEUTICAL JOINT-STOCK COMPANY
Nhon Trach 3 Industrial Park, Hiep Phuoc ward, Nhon Trach district, Dong Nai province
Tel: 02513 566 202 - Fax: 02513 566 203