- Symptomatic treatment of vertigo.
- Elderly suffering from loss of memory; vertigo, lack of concentration or alertness, changes of mood; deterioration in behaviour, personal negligence, dementia produced by multiple cerebral infarction.
- Acute ischemic stroke (particularly in patients with greater initial neurologic impairment who received treatment within 7 hours of stroke onset).
- Sickle cell anemia (piracetam has been reported to inhibit and reverse sickling in vitro and to produce benificial effects in patients with sickle cell anemia).
- Difficulty in reading children (used as supportive treatment)
- Used as an adjunct in the treatment of corticocerebralmyoclonia.
Specifications
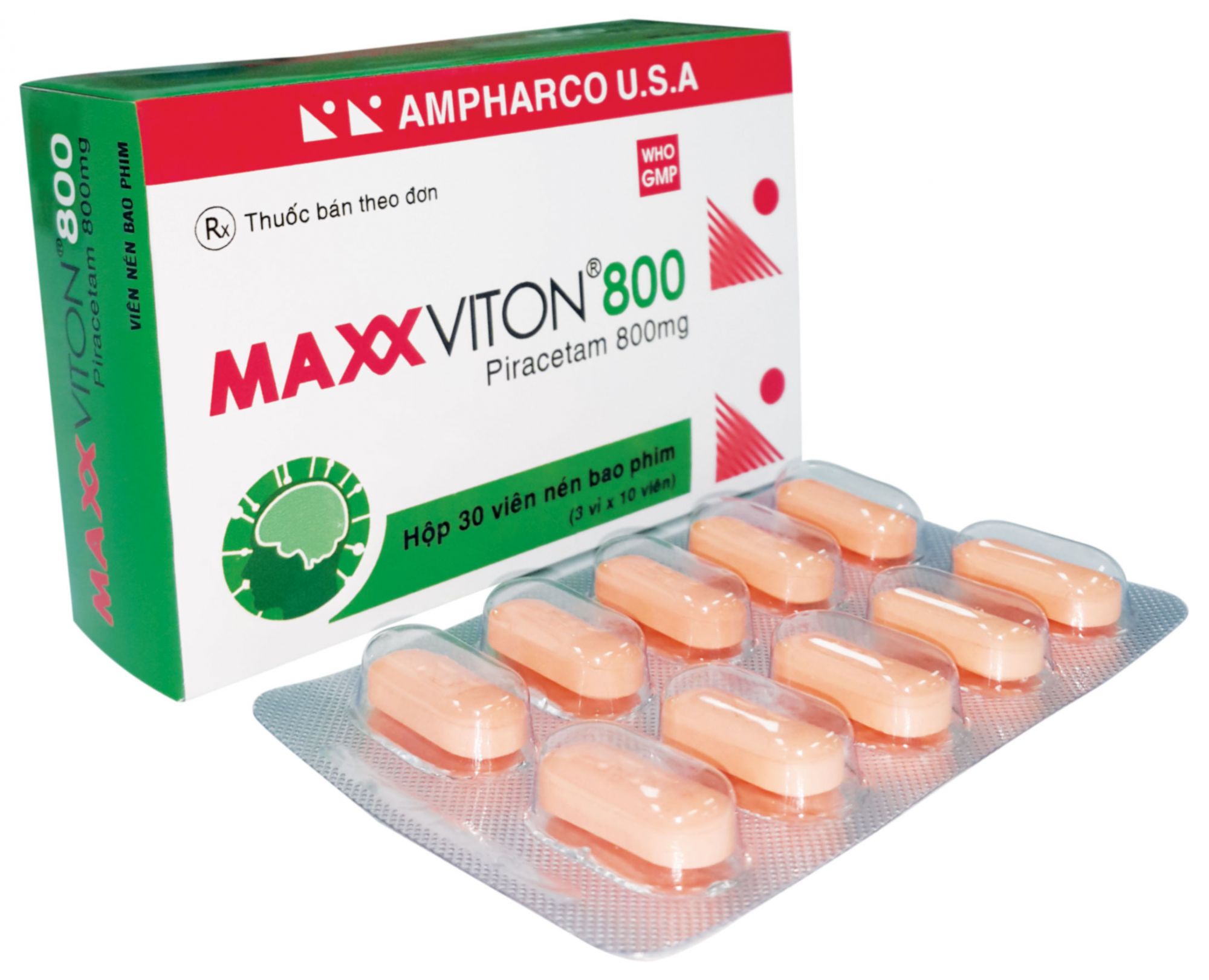
MAXXVITON® 800
COMPOSITION: Each film-coated tablet contains:
Piracetam..................................................................... 800 mg
Excipients: Povidone, Croscarmellose sodium, Microcrystalline cellulose, Magnesium stearate, Talc, Opadry II white, Sunset yellow lake, Brilliant blue lake.
INDICATIONS
- Symptomatic treatment of vertigo.
- Elderly suffering from loss of memory; vertigo, lack of concentration or alertness, changes of mood; deterioration in behaviour, personal negligence, dementia produced by multiple cerebral infarction.
- Acute ischemic stroke (particularly in patients with greater initial neurologic impairment who received treatment
within 7 hours of stroke onset). - Sickle cell anemia (piracetam has been reported to inhibit and reverse sickling in vitro and to produce benificial effects in patients with sickle cell anemia).
- Difficulty in reading children (used as supportive treatment).
- Used as an adjunct in the treatment of corticocerebral myoclonia.
DOSAGE AND ADMINISTRATION
The total daily dose is 30-160 mg/kg depending on the indication, administered orally twice daily or divided in 3-4 separate equal doses.
Long term treatment for psycho-organic syndroms in elderly patients: 1.2 -2.4 g daily, depending on individual conditions. The dose can be as high as 4.8 g daily during the initial weeks of therapy.
Alcoholism: 12 g daily during the initial withdrawal period. Subsequent maintenance therapy: 2.4 g daily given by mouth.
Cognitive deficits resulting from head injury (associated with vertigo or not): the initial dose is 9-12 g daily; the maintenance dose is 2.4 g given orally for a period of not less than 3 weeks.
Sickle cell anemia: 160 mg/kg daily divided in 4 equal doses.
Difficulty in reading children 8-13 years old: total dose 3,2 g/day in two sub-doses. It may be given in fruit juice or in some other drinks.
Corticocerebral myoclonia, a recommended protocol would be to begin treatment with 7.2 g of piracetam daily in 2-3 divided doses. This dosage should be increased by 4.8 g per day, every 3 or 4 days until obtaining a satisfactory response or a maximum dose of 20 g daily. Subsequently and depending on the results obtained, the dosage of other anti-myoclonic treatments should be reduced if possible.
CONTRAINDICATIONS
- Patients with known hypersensitivity to piracetam or any ingredient in the formulation.
- Patients with severe renal insufficiency (creatinine clearance < 20 ml/min).
- Patients with Huntington's disease.
- Patients with hepatic insufficiency.
WARNING and PRECAUTIONS
Since piracetam is eliminated via the kidneys, the increase in half life is directly related to the decrease of renal function and creatinine clearance. Special care must be taken when treating patients with renal insufficiency. Monitoring of renal function is recommended in these patients and in elderly patients.
When the creatinine clearance is < 60 ml/min or the serum creatinine is > 1.25 mg/100 ml, the dosage should
be adjusted:
Creatinine clearance 60-40 ml/min, serum creatinine 1.25-1.7 mg/100 ml (the half-life of piracetam is twice longer): 1/2 of normal dose.
Creatinine clearance 40-20 ml/min, serum creatinine 1.7-3.0 mg/100 ml (the half-life of piracetam is 25-42 hours): 1/4 of normal dose.
Safety in pregnancy
Piracetam may cross the placenta and is not recommended for pregnant women.
Safety in breast feeding
Piracetam is not recommended for breast feeding mothers.
PRESENTATION: Blister of 10 film-coated tablets; Box of 10 blisters.
Keep out of reach of children
Read the package insert carefully before use
Ask your doctor for further information
Use upon doctor’s prescription only
Manufactured and distributed by: AMPHARCO U.S.A – Pharmaceutical joint-stock company
Nhon Trach 3 Industrial Park, Hiep Phuoc Ward, Nhon Trach District, Dong Nai Province.
Tel: 02513 566 202 - Fax: 02513 566 203